
What about the D-Block Elements?įor the most part elements in the d-block have not been considered to be in the main group of elements on the periodic table. Unlike beryllium, a very transparent element, X-Rays can not penetrate barium so it is used to see the GI tract. It is a key component in the structures of living things like bones, teeth, shells, and exoskeletons. Calcium is even more abundant on Earth than magnesium. Magnesium is used in antacids, laxatives, and Epson salts. Magnesium is one of the most abundant alkaline earth metals. Atomic clocks use cesium because it can measure resonance frequencies accurately. The most common use for the element sodium (Na) is to season our food, in the form of table salt or sodium chloride (NaCl). The element lithium is a key factor in treating diseases like schizophrenia and bipolar disorder.

These main group elements and their compound are among the most economically important elements. Carbon is an essential element for all forms of life on Earth. Hydrogen, which is said to have been created during the hot Big Bang, makes up almost 70% of the universe. Like, oxygen and silicon, hydrogen and helium make up a majority of the universe. Oxygen and silicon make up a majority of the Earth’s crust. These elements make up 80% of Earth’s crust. The main group elements are most abundant in making up the earth and the universe. The main group elements are important for a few reasons, Earth and the Universe
The properties of these elements will vary because they span over a large portion of the periodic table of elements. Helium is excluded from the p-block, which starts at column 3A and ends at column 8A. These elements’ valence electrons are in the p orbital.

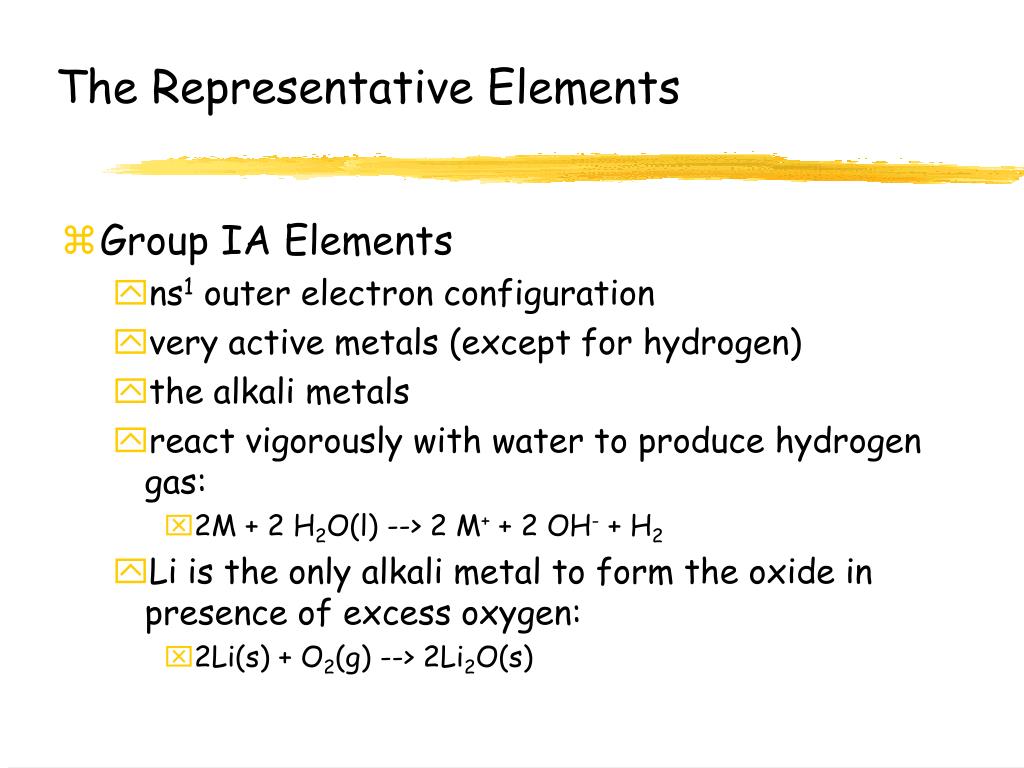
This block is on the right side of the periodic table and contains elements ranging in six different columns. S-block metals are highly electropositive.They tend to be soft with low boiling points.All s-block elements are highly reactive, except helium.



 0 kommentar(er)
0 kommentar(er)
